The pharmaceutical industry operates within some of the most demanding regulatory frameworks in the world. As digital transformation accelerates, organisations must safeguard the integrity, authenticity and confidentiality of electronic records. Regulatory frameworks now require this as standard practice.
Victoria Allen

Recent Posts
Ensure compliance in the pharmaceutical industry with SigningHub
Why MD Consents uses SigningHub: “Reliable, collaborative, professional”
Fertility clinics work within a highly sensitive sector which requires patient consent and comprehension. MD Consents is acutely familiar with these requirements, particularly when the company launched its fertilityconsent.com service.
How eSignatures provide a paperless solution for HR departments
Human Resources (HR) departments play a crucial role in companies. From recruiting top talent to managing employee benefits and confidential data, the paperwork required is vast and the need for security cannot be understated.
The power of eSignatures in real estate
The world is becoming increasingly digital. This blog explores how eSignatures are helping streamline the real estate industry and how SigningHub is helping estate agents overcome document workflow challenges.
Going digital: Government eSignature adoption
Our industry-focused blog series is back. Following the recent blog on banking this time we're discussing government eSignatures. In this blog, we’re exploring specific electronic signature initiatives and laws, and the challenges governments face.
9 common threats to your electronically signed documents
Our eBook, 9 common threats to your electronically signed documents, looks at the most common scenarios businesses could face and how to respond to them. It will help you understand how eSignatures stand up in a court of law with authenticity and integrity.
Can your signatures survive court scrutiny? Projects implementing eSignatures usually focus on the business benefits of cost-cutting, efficiency and improved user experience. However, the initial driver for eSigning projects is often compliance and adhering to regulations. Implementing legally binding signatures requires businesses to think of the potential threat scenarios and ensure countermeasures are in place to mitigate risks.
How to use SigningHub: apps and integrations
Designed to help businesses successfully digitise their business processes, SigningHub improves efficiency, boosts productivity, ensures security and saves time and money over the traditional signature process. This blog, the fourth and final in our ‘How to Use SigningHub’ series, explains how easy it is to use SigningHub's apps and integrations for your existing apps, software and platforms.
How to sign documents with SigningHub
By now, the convenience that online signing delivers to people and businesses is clear to most. Signing a document online with high levels of security can take a matter of seconds – eliminating the need for most to still use paper.
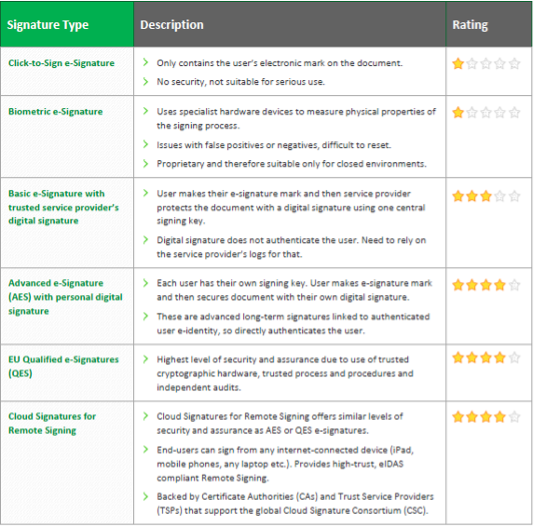
What is remote signing? Cloud signatures explained
The final blog in our series on the different types of eSignatures, this deep dive focuses on Cloud Signatures for Remote Signing and the details around this eSignature solution.
Increasing workplace productivity with eSignatures
The successful digitisation of business processes is not an easy undertaking. The paperless office is rapidly becoming the norm for most businesses.