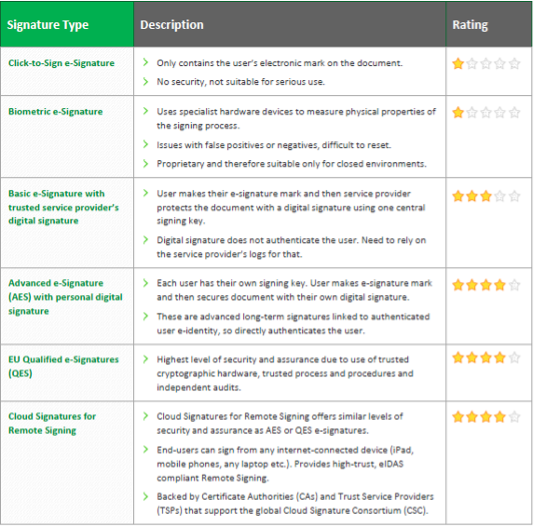
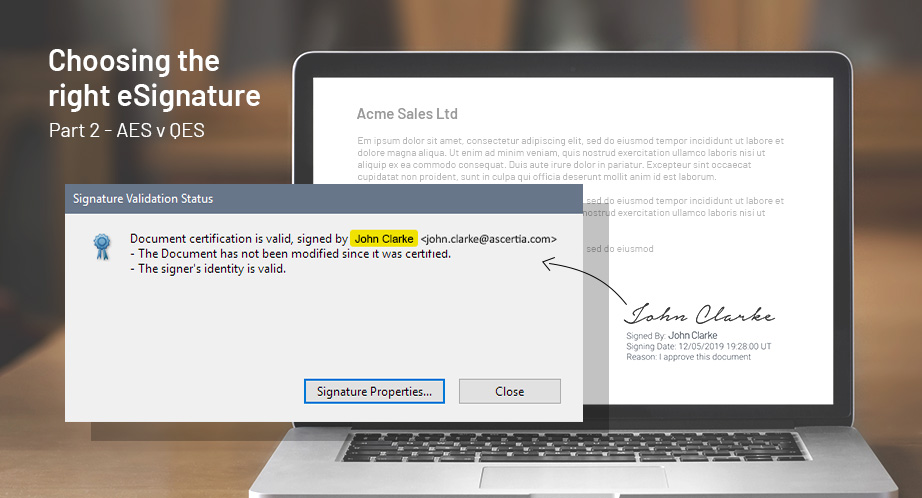
Our eBook, 9 common threats to your electronically signed documents, looks at the most common scenarios businesses could face and how to respond to them. It will help you understand how eSignatures stand up in a court of law with authenticity and integrity.
Can your signatures survive court scrutiny? Projects implementing eSignatures usually focus on the business benefits of cost-cutting, efficiency and improved user experience. However, the initial driver for eSigning projects is often compliance and adhering to regulations. Implementing legally binding signatures requires businesses to think of the potential threat scenarios and ensure countermeasures are in place to mitigate risks.